Abstract
Background The Molecular International Prognostic Scoring System (IPSS-M) is a newly developed risk stratification model for myelodysplastic syndromes (MDS) that expands on the original IPSS-R by including data on somatic oncogenic mutations across 31 gene families and weighing their impact on prognosis and outcomes. The discovery model demonstrated improved prognostic discrimination relative to the IPSS-R in regard to overall survival (OS), leukemia-free survival (LFS) and leukemic transformation, by substratifying pts into six risk categories, and led to restratification of 46% of cases. Adequate risk stratification in MDS is critical for therapeutic decision-making. Here we aimed to externally validate the findings of the original study using a large cohort of pts treated at a tertiary referral center.
Methods A total of 2355 MDS pts treated at Moffitt Cancer Center with available clinical and molecular data were analyzed. For estimation of the IPSS-M score, R version 4.2.1 was used to run batch-calculation using syntax code provided by the Papaemmanuil lab at MSKCC. The mean IPSS-M score was used as reference. Correlative analysis between IPSS-R and mean IPSS-M scores and outcome predictions was performed on LFS, OS and leukemic transformation. Time-to-event analyses were estimated using the Kaplan-Meier method and groups were compared by the log-rank test. We used Cox proportional hazards models for survival endpoints. For model discrimination we used Harrell's C concordance index.
Results We identified at least 4,327 driver point mutations involving up to 170 genes across 2355 pts. We identified at least one gene mutation in 83% of pts (1948). Median follow-up was 4.6 yrs (4.4-4.9).
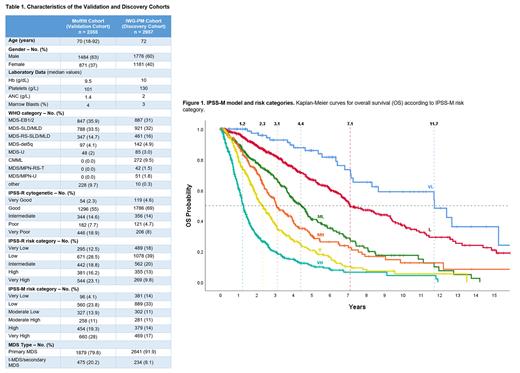
2333 pts (99%) were classified. Table 1 shows baseline clinical and molecular characteristics between validation and discovery cohorts. Using the IPSS-M risk stratification schema, pts in the validation cohort were classified as Very Low (96, 4% vs 14% in the discovery cohort), Low (560, 24% vs 32%), Moderate Low (327, 14% vs 11%), Moderate High (258, 11% vs 11%), High (454, 19% vs 14%) and Very High (660, 28% vs 18%) (Fig. 1A). The validation cohort was comprised of sicker pts with higher risk disease per IPSS-M relative to the discovery cohort (47% vs 31%).
The IPSS-M score resulted in improved discrimination relative to the IPSS-R regarding LFS, OS and leukemic transformation as evidenced by a 2.3-, 2.0- and 1.5-point increase in C-index.
The IPSS-M categories showed significant separation across all examined endpoints. Median LFS and OS estimates were equivalent between validation and discovery cohorts. Median LFS was 12.3 (vs 9.7), 6.9 (6.0), 3.6 (4.1), 2.2 (2.3), 1.4 (1.7) and 0.5 (0.7) yrs from Very Low to Very High-risk categories (Fig. 1B-1C). Conversely, median OS was 11.7 (vs. 10.6), 7.1 (6.0), 4.4 (4.6), 3.1 (2.8), 2.3 (1.7) and 1.3 (1.0) yrs from VL to VH subgroups (Fig.1).
A five-to-five mapping between the IPSS-R and IPSS-M risk subgroups (from combining moderate-low (ML) and moderate-high (MH) into moderate), resulted in the restratification of 45% of pts (1056), equivalent to the discovery cohort (46%). Almost 3/4 of pts classified as IPSS-R very-low (VL) were upstaged (majority to IPSS-M low and ML/MH). Almost half of pts classified as IPSS-R intermediate shifted: 39% (174) were reclassified as IPSS-M high/very high (H/VH). Similarly, about 42% of pts classified as IPSS-R high were upstaged to IPSS-M VH. Almost 20% of VH risk pts were downstaged (majority to high risk). Most significant was the reclassification of 10.5% (244) of the validation cohort from IPSS-R lower and intermediate risk categories into IPSS-M higher risk strata. Contextually, the restratification of pts from IPSS-R intermediate to higher risk strata in IPSS-M was associated with notable differences in survival outcomes which were as poor as 0.5 yrs and as high as 6.9 yrs for those reclassified as LR (relative to 1.9 yrs).
Conclusion To our knowledge this is the first external validation of IPSS-M in a large cohort. Use of the IPSS-M model for the risk stratification of pts with MDS resulted in improved discrimination of LFS, OS and risk of leukemic transformation over the IPSS-R. Furthermore, it led to restratification of almost half of MDS pts and exposed important differences in survival outcomes. Generalized use of this tool will likely result in more accurate prognostic assessment and optimize therapeutic decision-making among MDS pts.
Disclosures
Tinsley-Vance:Abbvie: Consultancy; Novartis: Consultancy; Jazz: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; CTI: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau. Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Sweet:Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Syntrix Pharmaceuticals: Research Funding; berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Lancet:Astellas: Consultancy; Agios/Servio: Consultancy; Jazz: Consultancy; BerGenBio: Consultancy; Millenium Pharma/Takeda: Consultancy; ElevateBio Management: Consultancy; Daiichi Sankyo: Consultancy; Celgene/BMS: Research Funding; AbbVie: Consultancy; Syntrix Pharmaceuticals: Research Funding; Dava Oncology: Consultancy; Boxer Capital: Consultancy; Dedham Group: Consultancy; Jasper Therapeutics: Consultancy; Novartis: Consultancy; Servier: Consultancy. Padron:Taiho: Honoraria; Blueprint: Honoraria; Incyte: Research Funding; Syntrix Pharmaceuticals: Research Funding; Kura: Research Funding; BMS: Research Funding; Stemline: Honoraria. Sallman:Agios: Membership on an entity's Board of Directors or advisory committees; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Syntrix Pharmaceuticals: Research Funding; Lixte: Patents & Royalties: LB-100; Syndax: Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Intellia: Membership on an entity's Board of Directors or advisory committees; Nemucore: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Komrokji:Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Taiho: Honoraria, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Honoraria, Other, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI biopharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal